Abstract
Introduction: CLL is the most common type of leukemia among adults in the US, yet current data on treatment patterns in clinical practice are limited. In recent years, novel oral targeted therapies (e.g., ibrutinib, acalabrutinib, venetoclax) have been increasingly used instead of chemoimmunotherapy (CIT) as frontline treatment. Therefore, this study aims to provide an up-to-date description of real-world treatment patterns among patients diagnosed with CLL in the US.
Methods: Using retrospective data from the Optum Clinformatics DataMartTM database (01/2013-12/2021), patients with ≥2 medical encounters with a diagnosis code for CLL or small lymphocytic lymphoma (SLL) on different dates were selected; the earliest diagnosis date was defined as the index date. Patients were required to have ≥12 months of continuous enrollment pre-index date (i.e., baseline period) and be aged ≥18 years as of the index date. Patients with a diagnosis for mantle cell lymphoma or with evidence of anticancer therapy (antineoplastic, radiation, cell therapy) during the baseline period were excluded. The observation period spanned from the index date up to date of death, end of continuous enrollment, or end of data availability, whichever occurred first.
Lines of therapy (LOTs) were identified using an adapted algorithm developed from previously published studies. Each LOT spanned from treatment initiation up to discontinuation of all agents in the LOT (i.e., 90-day gap without any dispensing), a switch to another LOT, or the addition of a new agent. Kaplan-Meier analysis was used to evaluate median time to the first line (1L) and from 1L to second line (2L) to account for censoring. To describe longitudinal treatment patterns, analyses were stratified for patients with their first CLL diagnosis between 01/2014-12/2017 and 01/2018-12/2021.
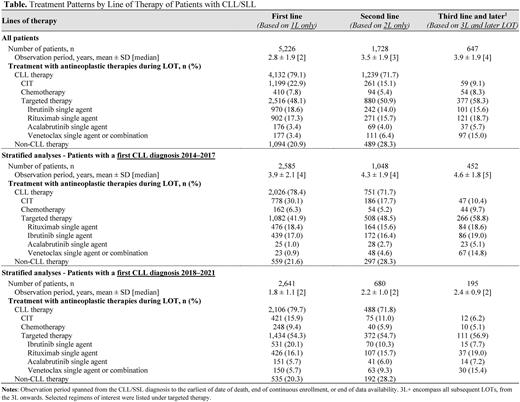
Results: Among 18,418 patients with CLL, the median age was 73 years and 43% were female. Patients had a mean Charlson comorbidity index score of 2.1. Analysis of treatment patterns (Table) showed that 5,226 patients (28%) were treated with ≥1 LOT and 1,728 (9%) of patients were treated with ≥2 LOT. Median time from index date to 1L initiation was more than 8 years (median not reached), while median time from 1L initiation to 2L initiation was 3.8 years. Of all patients who used antineoplastic therapies in 1L, most received targeted therapy (48%) or CIT (23%). Ibrutinib (19%), rituximab (17%), and bendamustine + rituximab (12%) were identified as the most common 1L regimens. In 2L, 51% of patients used targeted therapy and 15% used CIT. Rituximab (16%), ibrutinib (14%), and bendamustine + rituximab (8%) were identified as the most common 2L regimens.
Among patients diagnosed with CLL in 2014-2017 (N=2,585), 42% used targeted therapy and 30% used CIT in 1L (Table). The corresponding proportions for patients diagnosed with CLL in 2018-2021 (N=2,641) were 54% and 16%, respectively. The proportion of patients treated with CIT decreased with subsequent LOTs and even more so among those diagnosed with CLL in 2018-2021.
Conclusions: This long-term, real-world study showed a large delay between first CLL diagnosis and 1L treatment initiation, with a median time to treatment of more than 8 years. Targeted therapies were included in about half of treatment regimens in 1L and this proportion increased among patients diagnosed with CLL in more recent years (2018-2021). As use of novel therapies continues to increase and genetic testing becomes more widespread, further research will be needed to capture changes in CLL treatment paradigms and the subsequent impact on clinical outcomes.
Disclosures
Yang:Merck & Co., Inc.: Current Employment. Zanardo:Analysis Group, Inc.: Other: EZ is an employee of Analysis Group, Inc., a consulting company that has provided paid consulting services to Merck & Co., Inc., which funded the development and conduct of this study. Lejeune:Groupe d'analyse, Ltée: Other: DL is an employee of Groupe d'analyse, Ltée, a consulting company that has provided paid consulting services to Merck & Co., Inc., which funded the development and conduct of this study. De Nigris:Merck Sharp & Dohme: Current Employment. Sarpong:Merck & Co., Inc.: Current Employment. Farooqui:Merck & Co., Inc.: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Donzella:Merck & Co., Inc.: Current Employment. Laliberté:Groupe d'analyse, Ltée: Other: FL is an employee of Groupe d'analyse, Ltée, a consulting company that has provided paid consulting services to Merck & Co., Inc., which funded the development and conduct of this study; GSK: Research Funding.
OffLabel Disclosure:
Rituximab and bendamustine + rituximab were observed to be used by patients with CLL in the first and second lines of therapy (observational data).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal